AAV pathogenesis
The development of AAV is a complex and multifactorial autoimmune process2,3
The initial causes of AAV are currently unclear.3 Predisposing factors such as microbial infection, genetic influence, environmental agents and specific drugs are all fundamental to the development of AAV.2,3
Exposure to silica, pesticides, fumes, construction materials, hydrocarbon (cleaning agents, paint, diesel), drugs (propylthiouracil, hydralazine, D-penicillamine, cefotaxime, minocycline, anti-TNF agents, phenytoin) and certain psychoactive agents may all cause AAV.2,3
ANCA involvement
Loss of immune tolerance to ANCA antigens and development of ANCA by plasma cells1
ANCA are most commonly directed against the neutrophil lysosomal enzymes PR3 and MPO in GPA and MPA, respectively1-5
Neutrophils are primed
Neutrophils are primed by inflammatory cytokines (TNF-α, IL-1 and IL-18) produced in response to an infection or another event, with genetic predisposition also relevant2,6,7
ANCA antigens presented
Primed neutrophils present ANCA antigens (e.g. MPO and PR3) at their cell surface that bind to ANCA, resulting in neutrophil activation1,2,6,7
Inflammation mediators released
Activated neutrophils adhere to and penetrate the blood vessel wall, and release mediators of inflammation and cell injury, e.g. NETS1,2,6
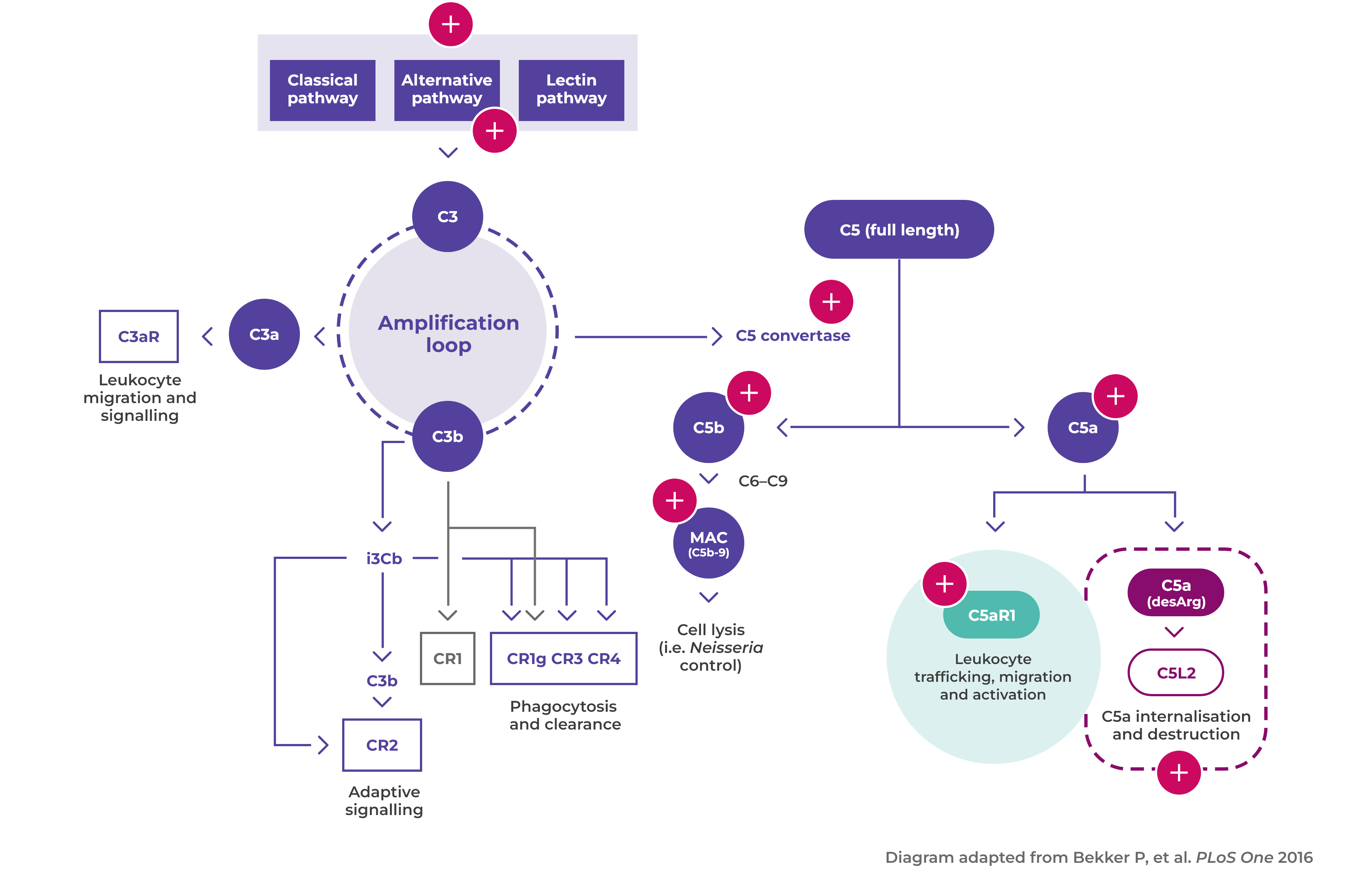
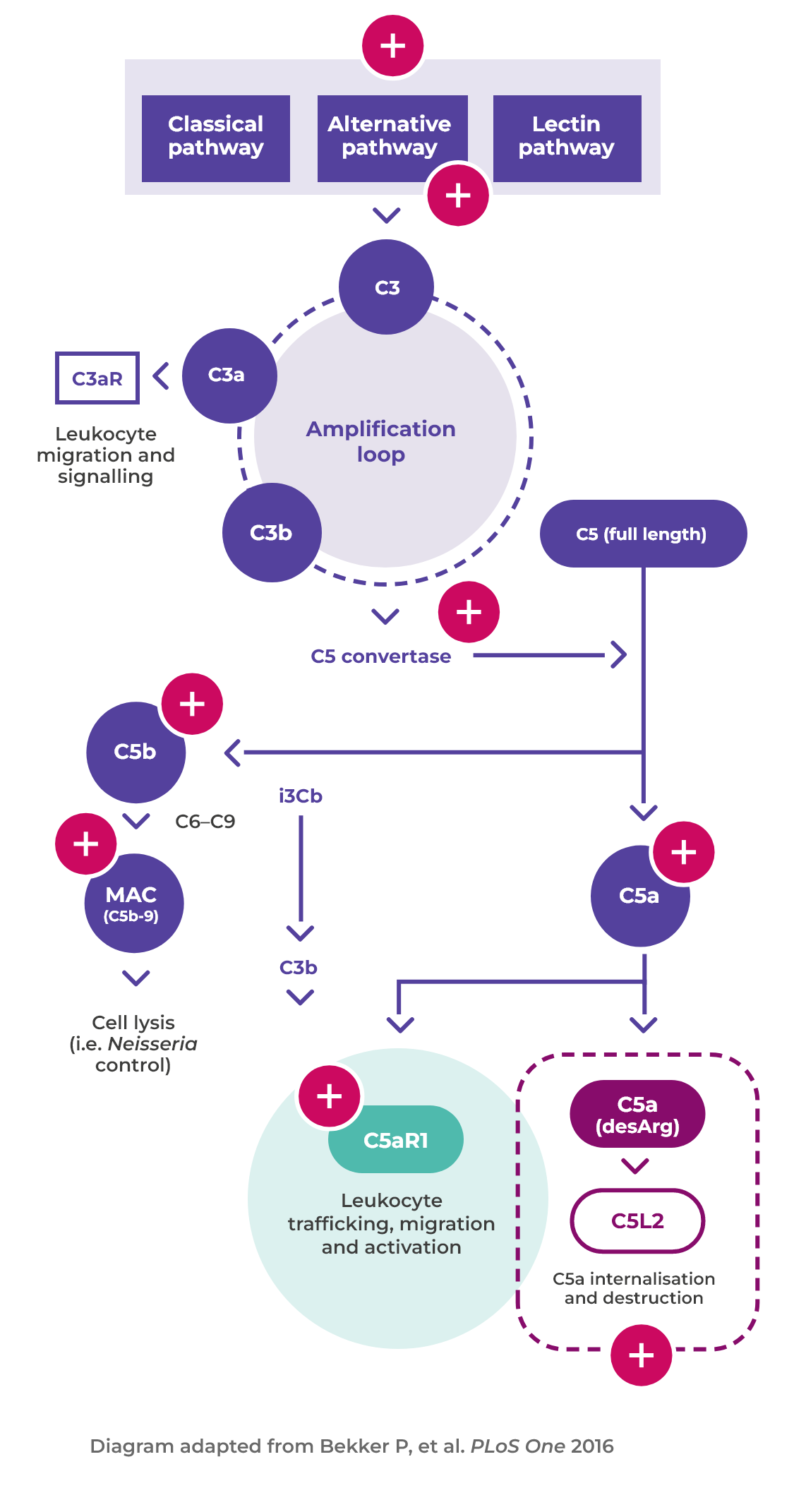
Alternative complement pathway activated
Activated neutrophils also release factors such as properdin that have an autocrine role in activating the alternative complement pathway, leading to the generation of C5a1,6
Binding of C5a to C5aR1
Binding of C5a to C5aR1 amplifies ANCA-induced inflammation and vascular damage6
Necrotising vasculitis
This process leads to necrotising vasculitis in small blood vessels6
Chronic inflammation
Over a few days, acute inflammation and necrosis are replaced by chronic inflammation and scarring6
The importance of complement and neutrophils in AAV activation
C5a plays a major role in the pathogenesis of AAV, amplifying ANCA-induced inflammation and vascular damage1

Introduction to AAV
AAV is a rare, severe small vessel vasculitis that affects multiple organs and has a high acute mortality risk1
Read more
Disease mechanism
The interaction between the activated alternative complement pathway, neutrophilsand C5a is at the heart of vasculitic damage in AAV2
Read more